Table of Contents
This short post details the technological and chemical processes to remove phosphorus from wastewater and other sources of water. Phosphorus is an element which known for its high flammability in elemental form, and for its essential role in biological processes and tissues.
What is Phosphorus
What is Phosphorus
Phosphorus is used widely as a water conditioning agent, although in recent year scarcity has reduced the quantities in which it is used domestically. Nevertheless, phosphorus is such a key element, and natural sources so low, that more investment into reuse is required for a sustainable closed loop.
Precipitation
The precipitation of phosphorus, normally called P-precipitation, is a chemical process in which salts are used to bind the phosphorus into a non-soluble form. This turns it into ‘flakes’ and makes for easier removal.
This process is based on the solubility of the salts that form. A soluble metal salt (such as magnesium chloride, or iron (II) sulfate, is dosed into the water. This causes the metal ions to bind to other ions in the solution, such as phosphate ions. Most metal phosphates are not very soluble, causing them to form flakes as they bind together. These flakes are then easily removed.
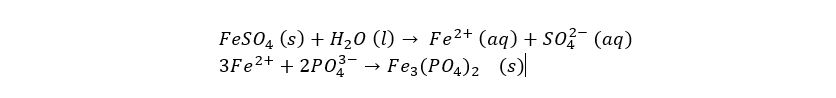
The chemical reaction above shows the iron(III) ions binding to dissolved phosphate to form iron(II)phosphate, also called ferrous phosphate or vivianite which can be used as a colouring agent or as fertiliser (Wiki link here).
Common Issues with Precipitation
The main problem is that these flakes end up in the sludge, which means the phosphorus is not easily separated. For this reason, p-precipitation is normally done after the main sludge removal stage of a sewage or other water treatment plant. This gives good removal
Additionally, some metal salts can also precipitate out other elements. For example, if a sulfate is used, such as iron sulfate, the sulfate ion can bind to lead in the wastewater to form lead sulfate which is insoluble. This causes lead to precipitate with the iron phosphate, contaminating the fertiliser. Sulfides, which are normally present in wastewater, also bind with almost all metals to form insoluble salts.
pH Based Removal
Phosphorus can be chemically removed from the sludge (solids) flow. This is done by using an acid solution on the sludge, which dissolves the C-containing compounds. These can then be strained out from the sludge, and mixed with a base, which precipitates the phosphorus salts out again.
This method is better for applications where a lot of the phosphorus is expected to the in the solid sludge, such as animal manure treatment.
Concluding Remarks
Phosphorus removal can be done in a variety of ways, and this process has been under scrutiny recently due to phosphorus’ essential function in fertiliser. Additionally, P discharge in our waterways causes environmental damage, by promoting high levesl of algal growth which remove oxygen from the water below, causing dead zones in seas, lakes, and rivers.
Current techniques rely heavily on chemical dosing, and separate processes. Although P removal is normally worth it, the industry needs to investigate more efficient ways to remove phosphorus for reuse to become more circular.
[…] Welsh Water, a UK drinking water company, has published a plan to invest £3.6 million ($4.6 million) in an upgrade to one of their sewage treatment plants. The technology to be used has not been published but it is likely that this is done by precipitation using metal salts. See more in our dedicated article on Phosphorus Removal. […]
[…] in a few decades.For more technical info on phosphorus and its removal from wastewater, read our article about it or see this MIT […]