Activated carbon (or: activated charcoal) is a widely used term for carbon that has been processed to make it highly effective at removing pollutants from water and air. In this article we will focus on the use of activated carbon (AC) for water treatment.
Intro – what is activated carbon
Activated Carbon (AC) is a type of nearly pure carbon that has excellent binding capabilities for many types of pollutant molecules such as aromatics and pesticides. The main structure of AC is made by activating blocks or particles of carbon that have been made out of charcoal from natural resources such as wood or bamboo, or from fossil coal or coke. There are two main processes to generate AC from coal or charcoal, using steam or chemicals.
AC is normally provided as a comaination of small parts or grains from 0.5 to 5mm in size and is then called granular activated carbon (GAC). Some important uses of activated carbon are water filtration, air filtration, mercury removal, and consumer uses like skin care additive and food additive. Most of the uses of AC rely on it’s high adsorptive qualities.
The high surface area of types of AC provides space for chemicals to bind to, removing them from the fluid flow. Different production processes create a number of types of activated carbon:
- Granular Activated Carbon (GAC): the most common type of AC used in many applications from water treatment to soil and air treatment.
- Powdered Activated Carbon (PAC): the leftover powdered AC from production or purposely ground down AC. Usually directly mixed with water or soil to adsorb chemicals or pollutants from it.
- Bead Activated Carbon (BAC): more circular than GAC, but the same principle applies. Usually smaller grain (bead) size than GAC, and used as lower pressure drop media in applications like columns.
- Treated types of AC: there are types of AC with a (bio)polymer coating that preserves the porosity and also protects the grains from breaking down. Woven AC is used in supercapacitors and odour adsorbers. Impregnated carbon is partially dosed with ions or molecules like silver, which has antibacterial properties, and other metal-dosed activated carbons are used for precise air quality control like museums.
A quick warning: we’re diving deep into chemistry here before discussing the production and uses of activated carbon!
Unique Characteristic and Uses
The main reason why activated carbon is so widely used is its very high surface area per volume (m2/m3). The surface of carbon has a chemical and physical ability to make certain other molecules stick to it by adsorption (see below for more info). This means that a small volume of AC can capture and hold a large amount of other molecules. The qualities of water make it such that it does not adsorp to the surface of AC, which means it passes through the solid, and only other molecules are left behind. Ions also don’t bind well to the surface of AC, so it can’t remove dissolved salts.
Some of the main pollutants of interest that are effectively removed using activated carbon are:
- Pesticides
- Medicines
- Certain types of aromatic hydrocarbons
Adsorption vs Absorption
‘Sorption’ is the physical binding of one chemical to another in a physiochemical way. The three types of sorption are absorption, adsorption (mind the ‘b’ and ‘d’ there) and ion exchange which is a type of exchange between electrolytes and will not be discussed in this article.
Adsorption
Adsorption (with a ‘d’) is the physical adhesion (clinging) of atoms or molecules to the surface of another material. The bulk solid, in this case carbon, has intermolecular forces that are neutral in the solid, but not neutral at the surface. This is because inside the solid, all atoms are surrounded by other atoms, evening out forces and moments, but at the surface, part of the atom or molecule is free to the outside world. This causes the surface to have some chemical or physical potential to attract molecules that then stick to its surface. In many cases this is by physisorption, which is the attraction of a molecule to a surface area by Van der Waals (VdW) Forces. These VdW Forces are a collection of forces that are not chemical (like ionic reactions), but are purely the forces that atoms exert on each other resulting from electrostatic charges or spontaneous polarisation in atoms. In short: quantum dynamics cause atoms to have small, random electric charges that can attract other particles.
At the surface area of a solid such as activated carbon, these VdW Forces are strong enough to dominate the ‘stickiness’ of the target molecules to the surface, while other molecules get a free pass.
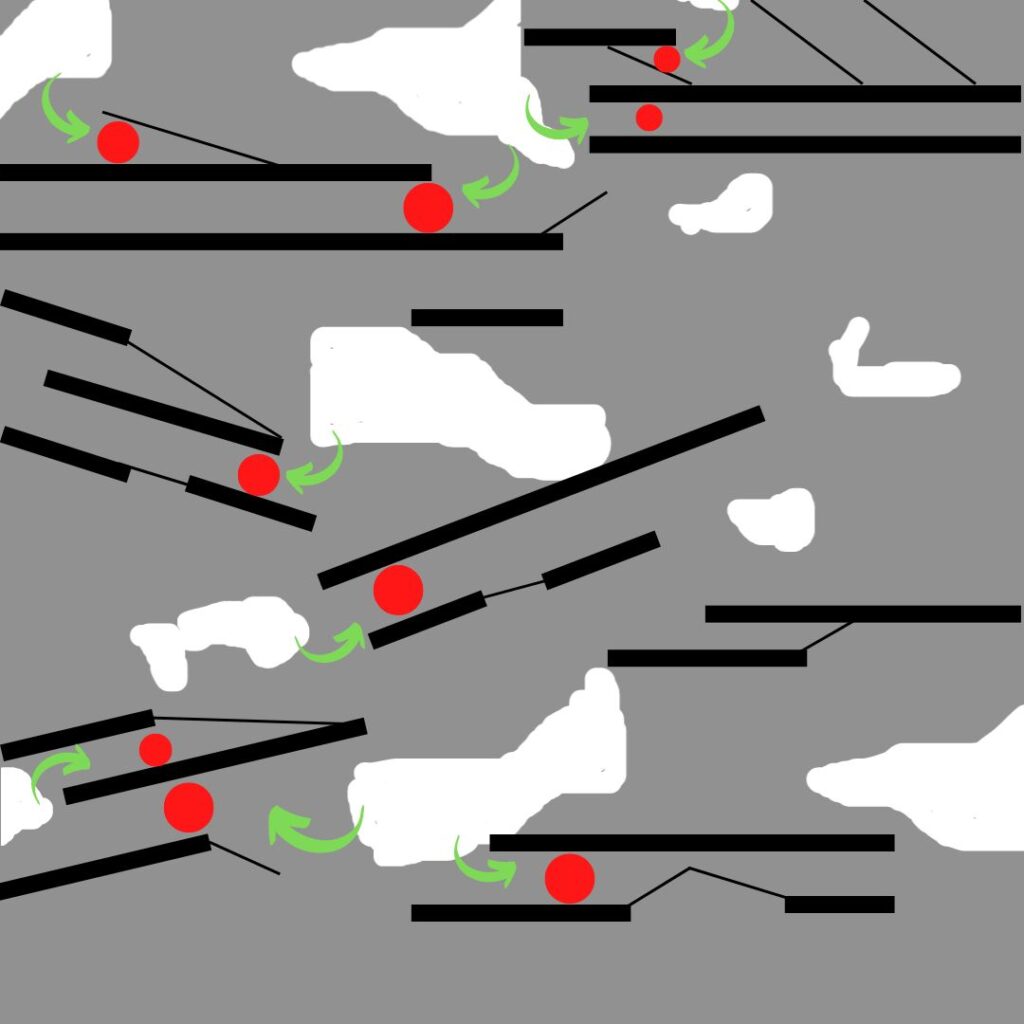
In this diagram the red dots represent pollutants that get adsorbed onto the pore structure of the activated carbon (black lines) after entering the material through the porosity of the bulk material (white gaps). The green arrows show the short path that the pollutants have to travel from the macropores to the micropores where they are eventually adsorbed and retained.
Absorption
Absorption (with a ‘b’) is a different type of sorption in which molecules of one type enter another type of material, either solid or liquid. Imagine a bubble of gas inside an amount of water. The bubble is in the gas phase, while the water is in the liquid phase. Now take all the single molecules in the gas, and using the chemical attraction between the water and the gas, hide each molecule of gas in the empty space between a few molecules of water. Now the bubble is dissolved into the water because instead of the molecules sticking together, they are absorbed in the space between the water molecules.
Properties and Tests
Activated Carbon has many properties and characteristics that define it. These are expressed in a number of ways, by performing different tests. The most used ones are:
- Iodine number; this is the capacity of the carbon to adsorb small iodine molecules from water. It is expressed in mg/g of adsorbed iodine and is done by simply mixing AC through a solution of iodine in water and measuring the concentrations before and after. The iodine number represents a relative capacity of the AC to adsorb smaller molecules.
- Molasses number; similar to the iodine number, but this number shows the capacity of the AC to adsorb larger molecules. It is done in a similar way to the iodine number test, but with molasses (a long carbon chain-type sugar), dissolved in water.
- Tannins test; similar to the two tests above, but this number shows the capacity of the AC to adsorb tannins, which are a combination of long and short chain molecules. It gives the overall capacity of micropores and mesopores of the carbon.
- Bulk density (apparent density); because of the grainy structure of AC, the density of the overall material is lower than that of the pure carbon that it is made of. This is due to the high amount of space, filled with air, that is inbetween the grains and inside the grains. The density of most pure carbons (actual density) is around 2000 kg/m3.
- Particle size distribution; the particles of the granular activated carbon (GAC) typically range from 0.4 to 1.2mm. Larger particles may saturate more slowly but also remove pollutants less efficiently. On the other hand, the larger a particle is the easier it is to keep it in a filter system without it washing away.
Surface Area and Porosity
Activated carbon, whether powdered, granular, or a block, is known for its very high relative surface area. One gram of activated carbon can have an internal surface area of more than 3000m2. An intuitive way to imagine this is to think about an enormous piece of paper that you crumple up to a ball more and more. There will be very small pores between the folds of the paper, so that the now very small ball of paper has a very high internal surface area. On this surface area, chemicals can ‘stick’.
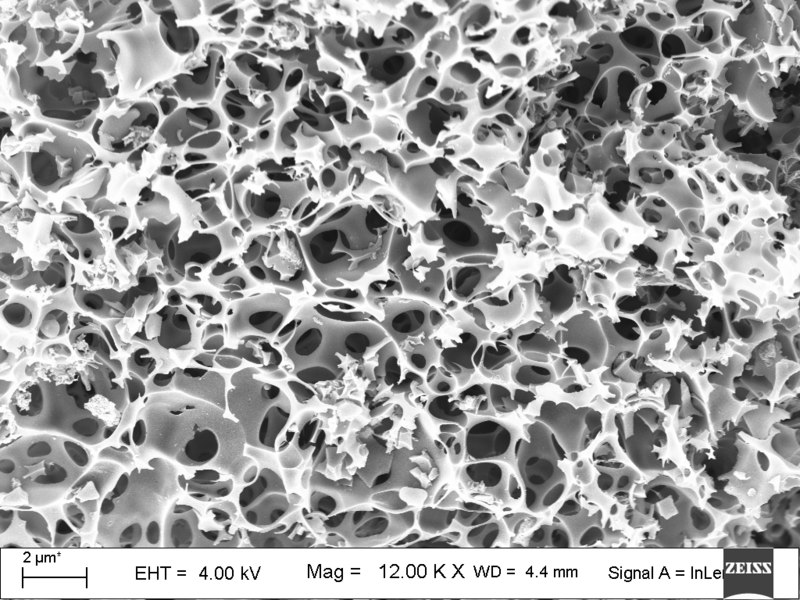
This image shows the structure of activated carbon made from tea leaves. It shows well the high porosity of the material, and how many small surfaces there are for chemicals to stick to.
Image by SomnathWiki007, CC BY 4.0, via Wikimedia Commons
Carbon Activation Process
There are a number of methods to turn regular coal or charcoal into activated carbon. The most standard method is to use high temperature steam, which ‘opens up’ the pores in the raw material, which is charcoal. Hot gas like air or nitrogen is then used to remove the steam. It is sometimes compared to making popcorn by using hot gas to expand a small grain into a larger ‘popped’ grain with higher surface area.
The activation process effectively removes water and other chemicals other than pure carbon from the original charcoal. This leaves only the ‘skeleton’ of the material. Because this happens at a very high temperature and without the presence of oxygen, the carbon does not burn but instead forms a thin and fragile network of atoms.
Chemical activation of AC works by using a strong acid or base, or some types of salts like calcium chloride or zinc chloride, and then heating the mixture. The strong chemical reactivity of the mixed substances force the same reaction in the carbon but at a lower temperature, creating the same porous structure of carbon atoms.
Actived Carbon Uses
Drinking water treatment is one of the most well-known uses of activated carbon. In larger scale plants, the carbon is added to the treatment water as powder and mixed so that it takes up the pollutants. In the next treatment steps it is filtered out of the water.
Carbon block filters are often used in smaller-scale systems. These blocks, shown on the side, have a cylindrical block of compressed grains of activated carbon in them. The water comes in through the outside of the clear vessel and is forced through the walls of the filter, past the carbon, to the inner collection tube. These can also be used on a large scale but the size shown here is a standard home or small industrial unit.
Image by Cschirp, CC BY-SA 3.0, via Wikimedia Commons

Air filtration
Carbon filtration is also a widely used method to purify air. The adsorption principles are the same as for water-based filtration. Activated carbon filters are used in household appliances like air purifiers and kitchen cooker hoods that recycle the air. The main factor that decreases the lifetime of these filters is the coating of microscopic droplets of fat that settles onto the GAC after a while, preventing the air from getting full contact with the carbon particles.
Recycling of Activated Carbon
Recycling of spent (used) activated carbon is often done with steam. The hot steam or other gas is run through the carbon mixture at temperatures over 500 degrees C (900 F), without oxygen present, which partially cracks and polymerises the organic substances that have been adsorbed by the carbon. This cracked material is then more easily desorbed (the opposite of adsorbed) from the carbon atom structure’s surface area.
The downside of regeneration is a partial loss of adsorbing capacity (around 5%-10% of the total capacity), which means AC is not infinitely recyclable. It is normally used and regenerated a number of times before being burned at high temperature furnaces to recover energy, or used as fuel for the regeneration process.
There are alternative methods of regeneration, using microwave energy, microbes, chemicals and solvents, or thermal or pressure swing treatments. These are all fairly self-explanatory, and replicate the effects of thermal recycling but at a lower temperature.
Closing Remarks
Activated Carbon has a highly porous structure which makes it perfectly suitable for adsorbing (not absorbing!) pollutants from fluid media like air and water. There are many applications for this material, with most of these focusing on removal of particular pollutants that remain after primary treatment in the case of water, and aerosolised chemicals and aromatics in air.
For more information on activated carbon and other filtration technology, see our Latest Posts.
[…] called AC, activated carbon is a method which we have covered in detail in a separate scientific article. The basic treatment principle is very different from RO: activated carbon is a type of carbon, […]
[…] has described the removal of PFAS from water using a new type of adsorbing media, similar to Activated Carbon but many times stronger. The working principle has not been published, although it is expected to […]
[…] US company AV (AqueoUS Vets) have launched a new cartride-type filter called Avloflo for the removal of PFAS, arsenic and other contaminants of emerging concern (CECs). The filter range has been designed for flows <110m3/hr (<400 gallon per minute) and comes in different sizes to deal with isolated rural supplies up to industrial flows. AV did not disclose the contents of their cartridges, although they specify using prefiltration to 1 micron. Since the company is known for their use of Activated Carbon (AC) and Ion-Exchange filters, it is to be expected that these cartridges use the same technology. Considering the broad applications recommended for these filters, from PFAS to arsenic, we hypothesize that it is AC. For more information on Activated Carbon see our in-depth article here. […]
I really value your piece of work, Great post.