This article highlights – on a high level – the different types of drinking water treatment. It can be used as a source for general information, but also contains links to in-depth articles and other sources.
Please let us know in the comments if there is any information missing.
Side note – this is a basic overview article with common drinking water treatment processes. Follow the links to detailed and scientific descriptions of each process for in-depth information.

Intro
Treatment of drinking water (or potable water) is a crucial part of a community’s infrastructure. It ensures safe, potable water is available when required. In most countries, tap water is not potable as it may contain levels of contaminants higher than safe limits. Worldwide safe drinking water guidelines are set by the WHO (World Health Organisation), the health department of the UN (link). The WHO also publishes maximum levels of pollutants that can be present in drinking water.
Purely looking at treatment, there are many ways to improve the quality of water coming from a source. Common sources include ground water (wells), aquifers, rivers or lakes, but the water from these sources can often be polluted and unsafe to drink. Most drinking water treatment processes aim to do the following:
- Simple
- Remove solids from the water, such as sand or organic matter;
- Remove some volatile components – such as benzene.
- Advanced
- Reduce the amount of dissolved salts;
- Reduce the amount of bacteria or other microorganisms;
- Remove heavy metals – such as lead and arsenic;
Treatment methods
All drinking water treatment methods boil down -pun intended- to do the steps listed above. This can be done with an advanced treatment plant consisting of multiple steps, or in many simple ways for small scale production. Let’s go over the main types.
Typical three-step water treatment
This is the standard treatment method for most major drinking water production facilities throughout the world.
The first step is always a kind of filter to remove large objects like leaves and sticks. This leaves only small particle in the water. A coagulant is added after this, to bind these remaining small particles together into slightly larger particles. This is then dosed with a flocculant which is a chemical that binds multiple particles into larger flakes (or flocs). This makes it easier to remove them as the heavier particle will sink to the bottom. Typical coagulants are sulphates of metals, like iron sulphate (Fe2(SO4)3) or aluminium sulphate (Al2(SO4)3).
The most widely used flocculants are polymers, which use their long chains to bind the small particles into larger flocs. Note that this is not the same type of polymer as the polymers we use for the production of plastics. The flocs are usually removed using scrapers on the bottom of a basin, where the low flowrate of the water allows the flocs to sink down. Click here for more information on types of polymers, by the University of North Texas. More detail on the science behind coagulation from WCS Group, here.

Secondly, the process water goes through a set of sand filters. The water is first pumped to the top of a large filter ‘box’, filled with course sand. It drains quickly through this filter and leaves behind any particles that didn’t sink down in the flocculation stage.
After this, a second sand filter is used with much finer grain sand. The water is pumped through this filter, sometimes under pressure, to remove the last particles that are still remaining in the water. The filter’s design doesn’t allow the sand to escape with the water. Read our article on sand filtration for more information on this topic.
Finally, chemicals are used to reduce the amount of microorganisms such as bacteria in the water, and sometimes to remove heavy metals or other chemical contaminants. A few methods used for this are carbon filters, ozonation, and ion exchange.
Carbon filters are made of pure, very porous carbon. The structure of this carbon allows it to adsorb chemical contaminants. Until it reaches it capacity, and as long as the flowrates are not too high, carbon filters can remove close to 100% of contaminants.
Ozonation is simply bubbling pure ozone through the drinking water. Ozone is an aggressive oxidizing agent, which means it can easily let other chemicals react with oxygen. It does this by ‘breaking off’ an oxygen ion which easily binds with heavy metals and organic molecules such as pesticides, but also bacteria and viruses.
Ion exchange is a more complicated system that deserves its own post, but the basics are simple: a filter that consists certain types of salts is used to force the exchange of ions from the wastewater with ions from the filter. This way, harmful salts and metals can be removed from the water and replaced with harmless ions such as sodium.
Many countries add chlorine or hypochlorite to drinking water to reduce the amount of bacteria that can grow in the water during storage and while in pipes. Chlorine is effective and doesn’t leave the water to taste like chlorine because it evaporates easily from the water. Hypochlorite, usually as sodium salt, is much more stable in the water for longer which means it is the preferred method in some countries. Unfortunately this does leave traces in the water that cause it to have the distinct chlorine smell.
Membrane treatment
A widely used technology for the production of potable water is membrane treatment. There are many types of membranes and they deserve their own post. But here we will go through the basic process of potable water production from sea water using Reverse Osmosis (RO) membranes. This works by putting pressure on a flow of salt (or otherwise contaminated) water, which pushes it across the RO membrane.
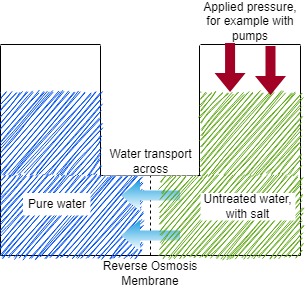
In essence, an RO membrane consists of a sheet of a strong, porous material such as polysulfone which is a strong polymer. On top of this, an ‘active layer’ is deposited which consists of a denser polymer such as polyamide (PA) or sometimes cellulose triacetate (CTA). The active layer has extremely small pores that only let water molecules pass. If pressurised water is forced against the active layer of the membrane, only the water will pass through and contaminants and salt will stay behind. This way, fresh water is produced without any salts or dissolved solids remaining.
The pores on a PA or CTA membrane do not work the same way as a filter. A simple filter works by excluding pollutants based on their size. The pores in the active layer of a reverse osmosis membrane are so small that even ions (dissolved salts) are left behind. Water is allowed through the membrane because of the small size of its molecules, but due to the water moving past the membrane the small pores also have a very low electrical charge. This charge is enough to block ions from passing, but allows water through. If you are interested in the transport phenomena across RO membranes, this link is a good starting point.
There is much more to RO membranes than this, and we encourage you to find out more in the dedicated membrane science part of our website!
Evaporation
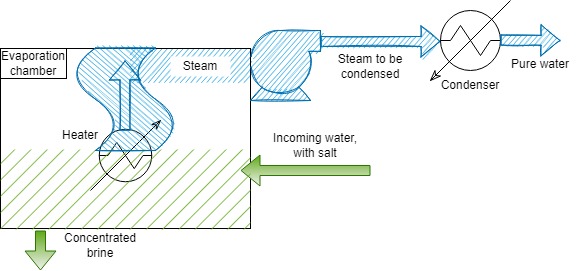
Evaporation and condensation is a popular – although old – method of producing drinking water. The concept is simple: heat water up until it evaporates, then capture the steam and cool that down until it condenses back into water. This is done both for seawater and freshwater, with the former being the most popular source.
The heating and evaporation of water requires a lot of energy, which is normally supplied by gas or oil. In some cases, o increase the efficiency of the process, the hot water is put in a chamber under low pressure. This lowers the boiling point, which reduces the heating required, making it cheaper and less environmentally damaging to treat the water. In most cases however, this type of water treatment is combined with a source of waste heat. There are many plants that produce elctricity and use the leftover heat to evaporate and condense water. A good example is the Umm Al Nar plant in Abu Dhabi, which generates 2,400 MW of electrical power and also produces 398,000 cubic metres of water per day (m3 d-1). That is enough to power 720,000 UK homes and provide water for 800,000 people in Abu Dhabi (or almost 3 million people in the UK!)
Closing remarks
There are many ways to treat water to make it suitable for drinking, but the one thing they have in common is that they use energy and other resources. This makes drinking water a valuable product, even in countries where there seems to be enough fresh water, like here in Scotland.
Many different technologies exist, and keep improving every year to be more sustainable and efficient. But this can only happen if the industry and the general population help by reducing water usage where possible. This will hopefully lead us to a future where there is enough water for everyone, even in countries where it is more difficult to treat it.
[…] flocculants, for small solids that are more readily removed after flocculation. See our article on water treatment basics or our dictionary for more information about […]
Great article! I found your perspective on this topic both enlightening and thought-provoking. The way you break down complex ideas into understandable insights is truly commendable. It’s interesting to see how these developments could shape our future. I’m particularly intrigued by your point about potential challenges and would love to dive deeper into that.
For those who are interested in exploring this topic further, I recommend checking out this resource for more detailed information: —. It offers additional insights that complement what’s discussed here.
Looking forward to hearing others’ thoughts and continuing this discussion. Thanks for sharing such valuable information!
Great article! I appreciate the clear and insightful perspective you’ve shared. It’s fascinating to see how this topic is developing. For those interested in diving deeper, I found an excellent resource that expands on these ideas: check it out here. Looking forward to hearing others’ thoughts and continuing the discussion!